Environment & Energy
Related: About this forumExtraction of Uranium in Supercritical Carbon Dioxide Using Incinerable Reagents
The paper I'll briefly discuss in this post is this one: Direct, Acid-Free Uranium Purification from Solid Analytical Waste by Supercritical Carbon Dioxide Extraction with Incinerable N,N-Dialkyl Amide Adducts, Avinash S. Kanekar, Shiny S. Kumar, Ankita Rao, and Sangita Dhara Industrial & Engineering Chemistry Research 2024 63 (48), 21001-21008.
Recently I remarked in a comment here that the flowback water from fracking operations in Pennsylvania is far more radioactive than seawater outside of the Fukushima reactor, Fukushima fetishes being the focus of antinuke stupidity that has resulted in the ongoing and accelerating destruction of the planetary atmosphere. I remarked that this is because the dangerous mining of dangerous natural gas takes place in a geological formation known as the Reading Prong, which is a fairly rich uranium ore. Fracking operations liberate radium and radon in the uranium decay pathway, these elements being in secular equilibrium with the parent uranium. Further I suggested, that once the fracking operations, which are destroying the atmosphere resulting in the extreme global heating we now experience, the uranium might be extracted using supercritical CO2 (SCCO2) as the solvent. (A current approach to uranium mining without the use of digging equipment is known as ISL (In Situ leaching) which is practiced in Kazakhstan, albeit using water, not SCCO2 as the solvent.
The paper referenced is lab scale, and scale up to industrial level may be problematic, but it's worth considering if not because we need, over the long term, in a sane world as opposed to the one in which we live, to mine uranium - in a fast neutron spectrum, the uranium and thorium already mined might supply all the world's energy needs for many centuries - but as a means of remediating uranium formations through which groundwater used for drinking and irrigation purposes passes.
Note that SCCO2 is an opportunity to put the dangerous fossil fuel waste CO2 to use, and it also notable that in such a case, as opposed to fracking operations, radium would not be extracted but rather rendered insoluble as the carbonate.
Some text from the paper's introduction:
Supercritical carbon dioxide (SC CO2), (10) modified with suitable extractants, is a lucrative option for metal ion extraction owing to its moderate critical constants (Pc = 72.8 atm., and Tc = 304.1 K), radiochemical stability, commercial availability, and nontoxicity. Due to its low viscosity and high diffusivity, SC CO2 extraction finds crucial applications for direct recovery of metal ions from solid matrices, thus limiting the number of process steps and secondary waste generation. Also, under ambient pressure and temperature conditions, CO2 gets converted to gas, leading to collection of a compact, concentrated metal-complex extract. N,N-dialkyl amides, by virtue of being incinerable and having tunable extraction properties with varying alkyl chain, have been investigated widely for solvent extraction studies. (10−15) Few studies on SC CO2 extraction of uranium and thorium, especially from nitric acid medium, using amides have been reported from our group. (16−18) Interestingly, direct extraction studies from crude solid matrices using adducts of extractants, especially tributyl phosphate (TBP), provide a unique opportunity (19) and have been explored by us. (20,21) N,N-dialkyl amides have been demonstrated to have higher selectivity for uranium over TBP. (22,23) However, systematic studies exploring amide adducts for extraction of uranium from crude matrices is lacking.
To the best of our knowledge, this is a first detailed investigation of the usage of adducts of N,N-dialkyl amides, viz. N,N-dibutyl octanamide (DBOA), N,N-dihexyl octanamide (DHOA), and N,N-dibutyl-2-ethyl hexanamide (DBEHA), for direct supercritical carbon dioxide extraction of uranium from actual solid waste matrices (W1 and W2). The physical properties (density and viscosity at different temperatures) and chemical composition (water and nitric acid contents) of the adducts were investigated. Isotherms for SC CO2 extraction of U from UO2 using the DBOA adduct were obtained to optimize the pressure and temperature conditions. The SC CO2 extraction efficiency of uranium from W1 and W2 as well as purification from other impurities was examined. The results were compared with the adduct of TBP. The feasibility of direct precipitation, without stripping, of uranium by ammonia addition was explored from the concentrated SC CO2 extracts for several runs. The precipitates obtained as such and heated to 773 K were characterized...
Some figures from the text:
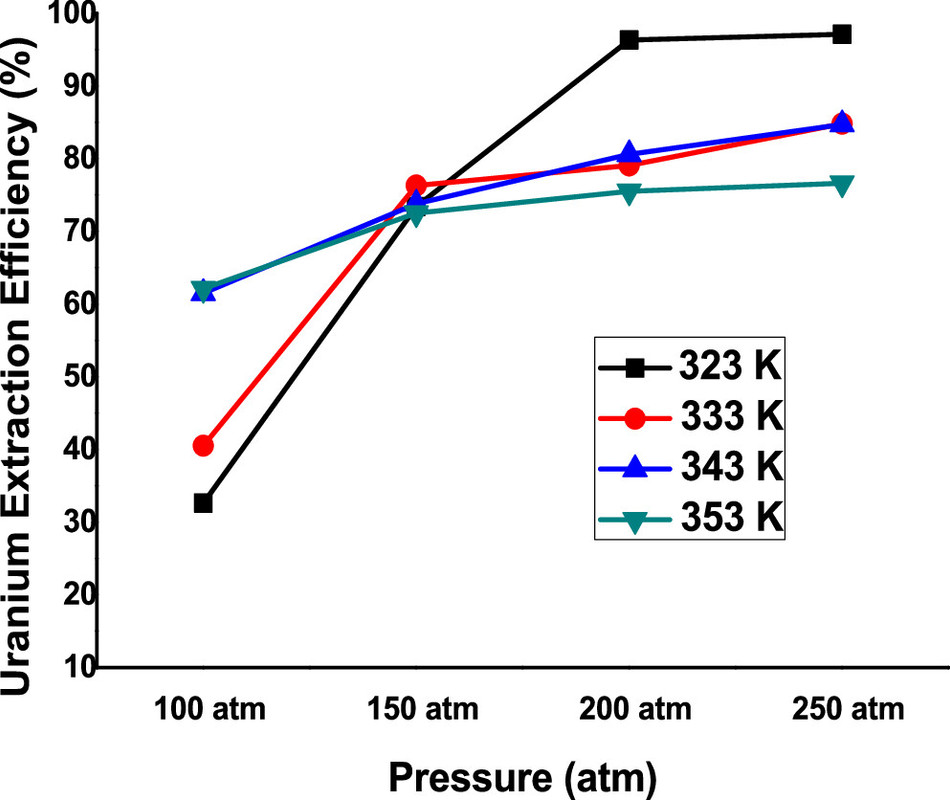
The caption:
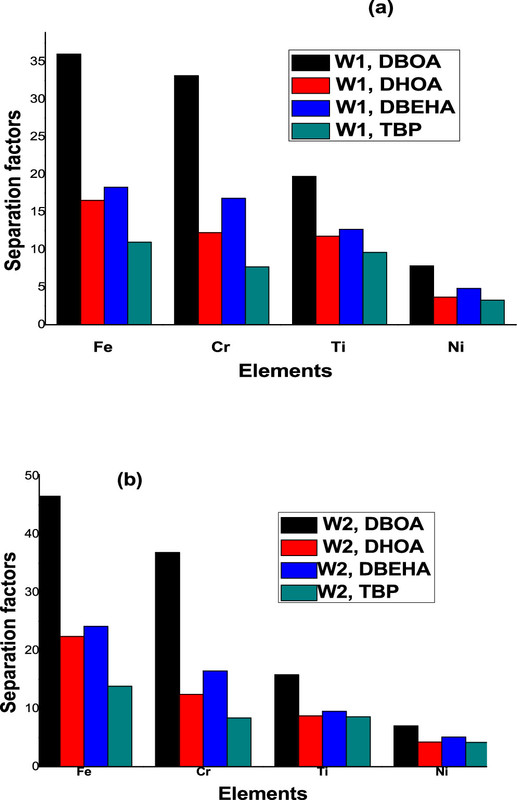
The caption:
Note: That in SCCO2 mining operations, depending on economics, selectivity toward metals other than uranium may not be a good thing.
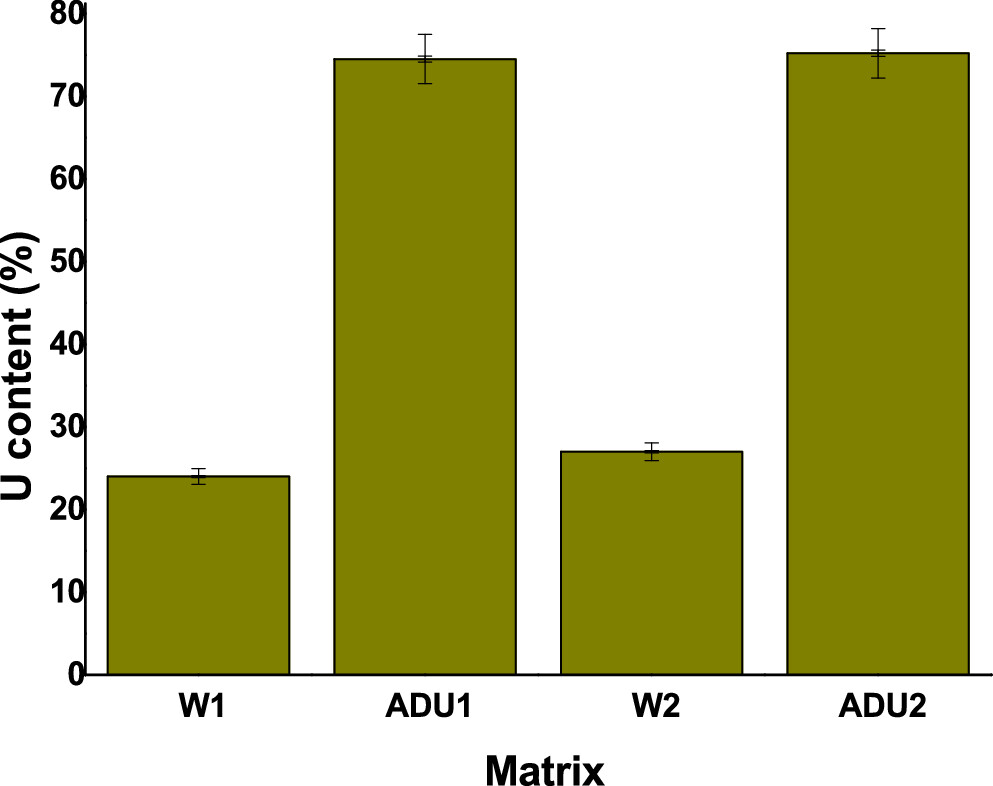
The caption:
In general, with such chemistry being utilized on an industrial scale in ISL settings, this would be an example of CCU, carbon capture and utilization, although it is unlikely that it would make much of a dent in the destruction to the atmosphere wrought by the dangerous fossil fuel waste CO2. Small amounts of the CO2 solvent would be geologically sequestered as carbonates under these condition, but nothing to generate much excitement. It is likely that the operation might also remove some of the fracking chemicals left in the formation by gas extraction. These would need to be treated in some way. The rocks shattered by fracking might perform far better, owing to particle size, in a uranium extraction operation.
From the paper's conclusions:
Have a nice weekend.
eppur_se_muova
(41,148 posts)(Not to be taken for granted. As one synthetic organic chemistry postdoc remarked, "Scale-up is fuck-up." Problems reliably emerge as the scale increases.)
NNadir
(37,392 posts)Last edited Sat Jan 11, 2025, 06:46 PM - Edit history (1)
...scientists, often under appreciated for what they do.
There is a risk that I see with this chemistry which may not be widely appreciated, which is the potential for the formation of N-nitrosamines, which are often a risk wherever a synthetic pathway involves secondary amines on scale. These can be managed, generally, but they are powerful carcinogens; they were discovered in the drug Valsartan (which I take) as a result of an environmentally and economically wise effort to recycle dimethylformamide, which as I'm sure you know, is a widely used solvent.
I would think that one would want to know the environmental fate of these molecules, but I think the effort is worth pursuing. There are. I would think that there are a multitude of organisms that can metabolize these, but I'm in no way certain. To the extent that the amides hydrolyze, the resulting amines would tend to retain CO2 even in a geological formation, I'd tend to believe.
eppur_se_muova
(41,148 posts)That sure surprised me. Dimethylamine is not surprising as an impurity, but that bacteria happily oxidize it to a nitrosamine was (to me -- I'm not very biochem-oriented).