Capturing carbon from the air just got easier
A new type of porous material called a covalent organic framework quickly sucks up carbon dioxide from ambient air
By Robert Sanders
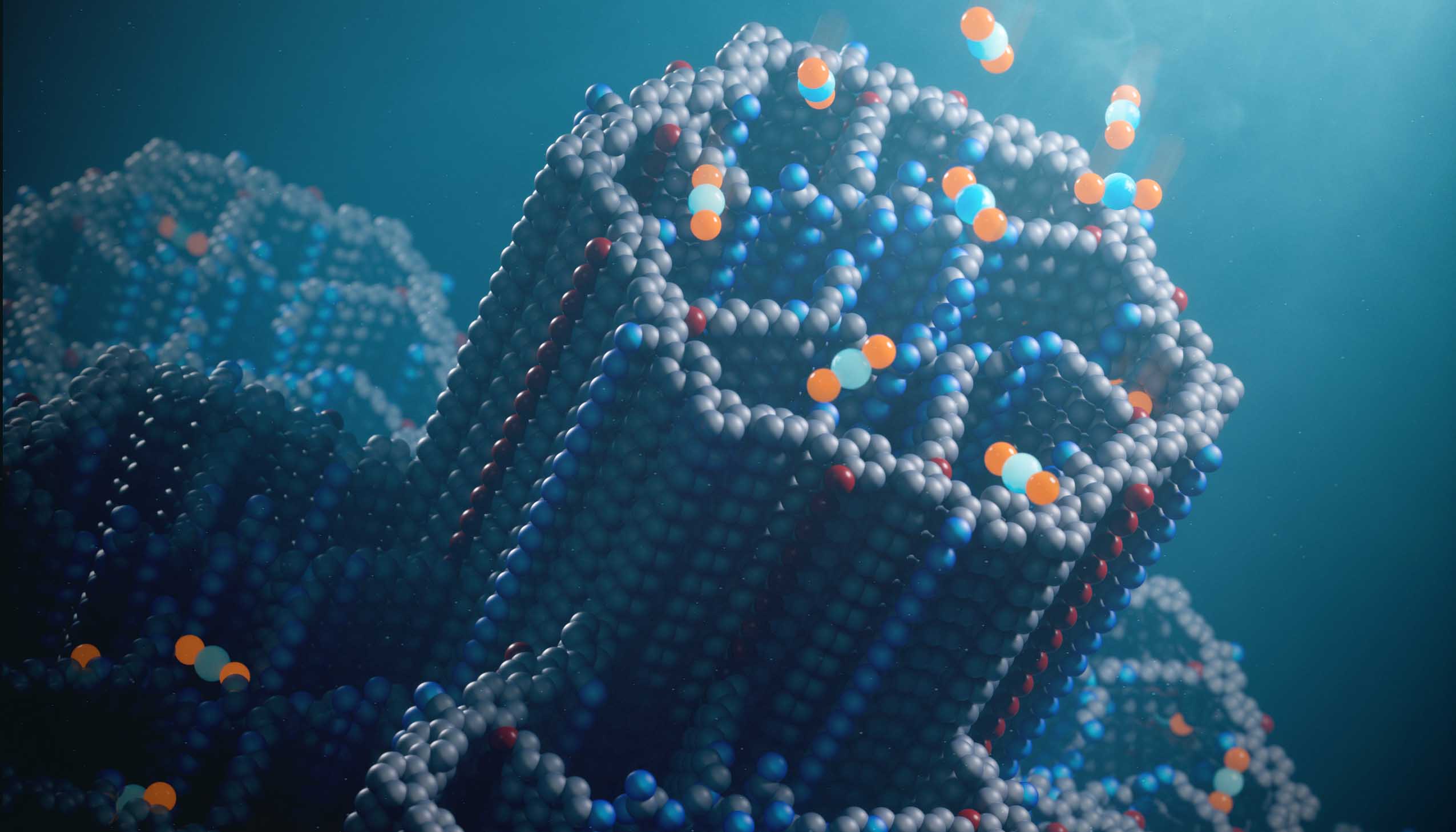
The new porous material for capturing carbon dioxide, called a covalent organic framework (COF), has hexagonal channels decorated with polyamines that efficiently bind carbon dioxide molecules (blue and orange balls) at concentrations found in ambient air.
Chaoyang Zhao
October 23, 2024
Capturing and storing the carbon dioxide humans produce is key to lowering atmospheric greenhouse gases and slowing global warming, but today’s carbon capture technologies work well only for concentrated sources of carbon, such as power plant exhaust. The same methods cannot efficiently capture carbon dioxide from ambient air, where concentrations are hundreds of times lower than in flue gases.
Yet direct air capture, or DAC, is being counted on to reverse the rise of CO₂ levels, which have reached 426 parts per million (ppm), 50% higher than levels before the Industrial Revolution. Without it, according to the Intergovernmental Panel on Climate Change, we won’t reach humanity’s goal of limiting warming to 1.5 °C (2.7 °F) above preexisting global averages.
A new type of absorbing material developed by chemists at the University of California, Berkeley, could help get the world to negative emissions. The porous material — a covalent organic framework (COF) — captures CO₂ from ambient air without degradation by water or other contaminants, one of the limitations of existing DAC technologies.
“We took a powder of this material, put it in a tube, and we passed Berkeley air — just outdoor air — into the material to see how it would perform, and it was beautiful. It cleaned the air entirely of CO₂. Everything,” said
Omar Yaghi, the James and Neeltje Tretter Professor of Chemistry at UC Berkeley and senior author of a paper that will appear online Oct. 23 in the journal
Nature.
…

A vial of COF-999, which is yellow, with UC Berkeley’s landmark campanile in the background.
Zihui Zhou, UC Berkeley
…
https://doi.org/10.1038/s41586-024-08080-x
As VMA131Marine suggests, it’s promissing, but a
long way from “prime time” (from the
Nature article):
…
Outlook
We have shown how COFs with an olefin-linked backbone and covalently attached sorption sites, bringing ultrahigh chemical stability, can serve as excellent materials for capturing CO₂ from the air. Our studies demonstrate that this application in the open air is a marked advance towards achieving clean air. It is also clear that the present COF-999 is perhaps one of the first members of what we believe will be a large class of materials with a robust framework backbone that we expect will serve the general purpose of carbon capture very well. By applying this strategy, other reticular structures will have to be designed, examined and compared with COF-999 to further improve the capacity and performance. In the meantime, the scalability of this COF and the design of a practical device will be an important priority for future implementation of these materials.
…

